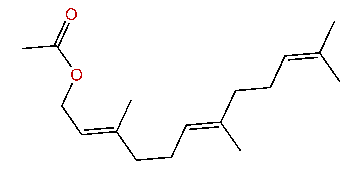
| (E,E)-3,7,11-Trimethyl-2,6,10-dodecatrienyl acetate |
| Formula: | C17H28O2 |
| CAS#: | 4128-17-0 |
| MW: | 264.4 |
[MS]
[ NMR ]
[ Kovats ]
[Behavioural function]
[Occurrence in flower]
|
|
Reference(s) for synthesis of (E,E)-3,7,11-Trimethyl-2,6,10-dodecatrienyl acetate [E,E-farnesyl acetate]
| Clark, J.S., Myatt, J., Wilson, C., Roberts, L., and Walshe, N. 2003. Exploration of the biomimetic synthesis of indole-diterpene mycotoxins: an unexpected cascade reaction during the attempted synthesis of emindole SB. Chem. Comm. 13:1546-1547. |
| |
| Takahashi, T., Nemoto, H., Kanda, Y., Tsuji, J., Fukazawa, Y., Okajima, T., and Fujise, Y. 1987. Macroring contraction methodology: 3. Total syntheses of costunolide and haageanolide using transannular [2,3]-Wittig rearrangement of 13-membered diallylic ethers as key reaction. Tetrahedron. 43:5499-5520. |
| |
| Yanagisawa, A., Noritake, Y., and Yamamoto, H. 1988. Chem. Lett. 1899-1902. |
| |
|







